Source prnewswire
Anaut Announces Japanese Regulatory Approval of AI-Powered Surgical Visualization Tool, Eureka α
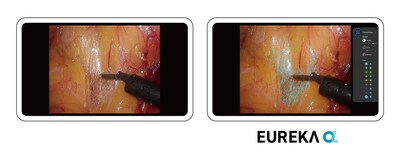
Anaut Inc., a pioneering developer in surgical support software led by CEO Dr. Nao Kobayashi, announced regulatory approval of its groundbreaking medical device, "Eureka α". This first-of-its-kind software device in Japan received approval from the Ministry of Health, Labour and Welfare on April 12, 2024 (approval number: 30600BZX00061000) and is set to transform surgical practices with its advanced artificial intelligence capabilities.Innovative Surgical Assistance Eureka α utilizes state-of-the-art AI to analyze real-time video from laparoscopic and robotic surgery, enhancing surgeons' accuracy by highlighting the dissection planes characterized by connective tissue. Connective tissue, critical in surgical navigation, acts as a key landmark during surgeries. Known variably as the "dissection plane" or "holy plane", its proper identification is crucial for safe and effective surgical outcomes. Visualization of connective tissue is critical in almost any surgical procedure in the abdomen, including those that involve the stomach, large intestine, and abdominal hernias Visualization of connective tissue is critical in almost any surgical procedure in the abdomen, including those that involve the stomach, large intestine, and abdominal herniasEureka α offers a secondary monitor view that differs from the primary surgical display to optimize the surgeon's visual field. Supported by expert-guided training data, the technology harnesses deep learning and computer vision technologies to improve surgical precision and safety.Development and Collaborative EffortsThe development of Eureka α was made possible by a robust partnership with over 20 of Japan's largest and most prominent academic research institutions and medical university hospitals. This initiative has also received backing from significant public organizations such as the Tokyo Metropolitan Government, the New Energy and Industrial Technology Development Organization (NEDO), the Japan External Trade Organization (JETRO), Kawasaki City, and others.A Leap Forward in Surgical StandardsJapan has long been recognized for its high safety standards in surgery, aided by technical accreditation systems and a focus on laparoscopic and robot-assisted techniques. Eureka α represents a pivotal advancement in supporting surgical operations, particularly benefiting young surgeons in mastering complex procedures through enhanced visual guidance and increased confidence in surgical performance.Future Prospects and Global AspirationsFollowing the success in Japan of its flagship product, Surgical Vision Eureka, which is employed in educational and research settings as a non-medical device, Anaut Inc. plans to expand the applications of its technology to include gynecological and urological surgeries. With no equivalent devices currently approved for clinical use outside of Japan, Eureka α has garnered international attention, paving the way for expansion into the US and global markets.About Anaut Inc.Founded in July 2020 in Tokyo, Japan by a team of surgeons, engineers, and business professionals, Anaut Inc. aims to merge cutting-edge engineering with surgical expertise to develop technologies that enhance patient care worldwide, including decreased complications and higher efficiency. "A nautical mission in the ocean of anatomy," the company continues to innovate at the intersection of technology and human health.ContactJames Y. GrosvenorGlobal Business DevelopmentAnaut Inc. [email protected]Visit our website at https://anaut-surg.com/en/ for further details.SOURCE Anaut Inc.

No Comments