Source prnewswire
Single Pass, Inc. Announces FDA Clearance of its Kronos Biopsy Closure Device
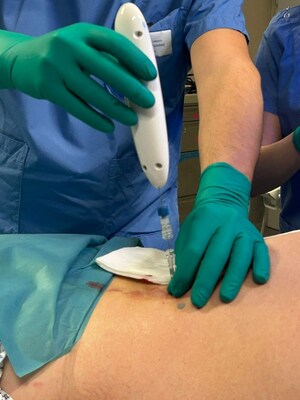
Single Pass, Inc. is proud to announce that its Class II Kronos biopsy closure device has received clearance from the U.S. Food and Drug Administration (FDA). The FDA has determined the device is substantially equivalent to predicate devices regarding its safety and effectiveness. Under the leadership of Bill Colone and its Board of Directors, the Single Pass team collaborated with their contract manufacturing partner, M4D, based in Lake Forest, CA to bring the Kronos device to market. The company previously achieved the CE Mark under the EU MDR regulations, as now the Kronos device is now commercially available globally. Under the leadership of Bill Colone and its Board of Directors, the Single Pass team collaborated with their contract manufacturing partner, M4D, based in Lake Forest, CA to bring the Kronos device to market. The company previously achieved the CE Mark under the EU MDR regulations, as now the Kronos device is now commercially available globally."We are thrilled to have received FDA clearance for our Kronos biopsy closure device," said Bill Colone, Co-founder, and CEO of Single Pass. "The Kronos device represents a significant advancement, offering healthcare professionals a reliable and efficient solution for post biopsy bleeding and improved patient care."Mermaid Medical, the US distribution partner, is prepared to initiate sales in the US, ensuring immediate national access to this groundbreaking technology. This strategic partnership will facilitate widespread coverage for this innovative and unique technology ultimately benefiting patients and healthcare providers across the country.For more information about Single Pass and its innovative medical devices, please visit https://www.singlepass.co/.About Single Pass: Single Pass is a medical device company dedicated to developing an innovative solution to post biopsy bleeding, enhance patient care, and improve clinical outcomes. With a focus on precision and reliability, Single Pass strives to revolutionize the healthcare landscape through cutting-edge technology by safely cauterizing deep tissue through a guide needle, promising swift, easy, and safe biopsy procedures.For media inquiries, please contact:Bill ColoneCEOSingle Pass+1-602-618-3377[email protected]SOURCE Single Pass, Inc.Single Pass, with the first and only biopsy closure cauterization device, is delighted to announce the successful completion of the first real- life...Single Pass, a leading provider of innovative medical devices, is pleased to announce an exclusive distribution agreement with Mermaid Medical Group, ...



No Comments